titanium number of valence electrons|Titanium Valence Electrons : Manila The 1st element in group-4 is titanium. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit. But in the case of transition elements, . Tingnan ang higit pa Our Batman: Arkham Origins trainer has 10 cheats and supports Steam. Cheat in this game and more with the WeMod app!
PH0 · What are the valence electrons of titanium? Chemistry Question
PH1 · What are the valence electrons of titanium? Chemistry Question
PH2 · Valences of the Elements Chemistry Table
PH3 · Valence Electrons Chart for All Elements
PH4 · Titanium Valence Electrons
PH5 · Titanium (Ti)
PH6 · Titanium
PH7 · How to Find the Valence Electrons for Titanium (Ti)?
PH8 · How to Find the Valence Electrons for Titanium (Ti)
PH9 · Determine valence electrons using the periodic table
PH10 · 10.6: Valence Electrons
ZIP code: 6014. IDD : area code +63 (0)32: Native languages: Cebuano: site: www.mandauecity.gov.ph: Mandaue, officially the City of Mandaue, or Mandaue City (Cebuano: Dakbayan sa Mandaue), is a 1st class highly urbanized city in the Central Visayas region of the Philippines. According to the 2020 census, it has a population of .
titanium number of valence electrons*******The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cation. The titanium atom donates two electrons in 4s orbital and two electrons in 3d orbital to convert to titanium ion(Ti4+). . Tingnan ang higit pa
The 1st element in group-4 is titanium. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit. But in the case of transition elements, . Tingnan ang higit patitanium number of valence electrons Titanium Valence Electrons Titanium (Ti) is an element located in Group 4 of the periodic table. The number of valence electrons an element has is determined . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency. The number of electrons in an unpaired state in . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not . Tingnan ang higit pa Mar 23, 2023
You may assume that the valences of the elements—the number of electrons .
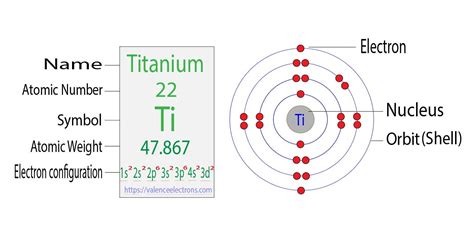
To find the number of valence electrons for Titanium (Ti) we need to look at its electron configuration. This is necessary because Ti is a transition metal (d block .titanium number of valence electrons Titanium in chemistry is known as the chemical element. It has the atomic number 22 and symbol as Ti. Flerovium Valence Electrons. Helium Valence Electrons. Plutonium Valence Electrons. Lithium .
Element Titanium (Ti), Group 4, Atomic Number 22, d-block, Mass 47.867. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main .
The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different .Titanium Valence Electrons Titanium has twenty-two protons and twenty-six neutrons in its nucleus, and twenty-two electrons in four shells. It is located in group four, period four and block d of the periodic .
Solution. Valence electrons: The electrons present in the outermost shell of an atom are known as the valence electrons. Titanium Ti: Titanium is a transition metal that .
Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding .

How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2) In fact, the number of valence electrons goes up by one for each step across a .The isotopes of titanium range in atomic mass from 38.01 u ( 38 Ti) to 62.99 u ( 63 Ti). Titanium-46 is composed of 22 protons, 24 neutrons, and 22 electrons. Titanium-47 is composed of 22 protons, 25 neutrons, and 22 electrons. Titanium-48 is composed of 22 protons, 26 neutrons, and 22 electrons. Titanium-49 is composed of 22 protons, 27 . Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the .
The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a valence of 1. Usually it’s oxidation state is +1, but it can lose the electron and have a valence of -1. The most stable oxidation state is one . The electron configuration for Titanium ion (Ti 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6. The number of valence electrons available for the Titanium atom is 4. Titanium is situated in the transition metal group and has an atomic number of 22. The orbital diagram for Titanium is drawn by following three principles – the Aufbau principle, Hund’s .
The "A/B System" group number indicates the number of valence electrons that are present in an atom. Neon (Ne) is located in Group 18, which is labeled as Group 8 A, using the "A/B System." Therefore, neon has 8 valence electrons. (This answer is consistent with the solution to Exercise \(\PageIndex{1}\text{a}\).)
And you have one more electron to worry about. And so that electron would go into a 3S orbital. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level.
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and .Titanium is a chemical element of the periodic table with chemical symbol Ti and atomic number 22 with an atomic weight of 47.8671 u and is classed as a transition metal. . Number of electrons: 22 e- . Valence electrons : .1.3: Valence electrons and open valences. A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's .For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed .
The Covalent Bond. Atoms can combine to achieve an octet of valence electrons by sharing electrons. Two fluorine atoms, for example, can form a stable F 2 molecule in which each atom has an octet of valence electrons by sharing a pair of electrons.. A pair of oxygen atoms can form an O 2 molecule in which each atom has a total of eight valence .
The Covalent Bond. Atoms can combine to achieve an octet of valence electrons by sharing electrons. Two fluorine atoms, for example, can form a stable F 2 molecule in which each atom has an octet of valence electrons by sharing a pair of electrons.. A pair of oxygen atoms can form an O 2 molecule in which each atom has a total of eight valence .
The valency of titanium is accurately four to attain stability. Valency is basically the integral properties of Titanium just like the other chemical element. The four valencies of Titanium states that it may either gain or lose 4 electrons. The outermost shell of Titanium holds the s and p electrons of this element.Titanium’s atomic symbol is Ti, and its atomic number is 22. It belongs to the transition metal group and can form compounds at an oxidation state of +4. Titanium’s abbreviated electron configuration is [Ar] 3d 2 4s 2. This shows which valence electrons can be lost in a bond with other elements.
Valence electron. The valence electron refers to the outermost electron in the outermost shell of an atom. Elements in the periodic table are arranged such that the elements in the same group will have the same valence electrons. For example, group 16 elements such as oxygen, sulfur, and selenium have 6 valence electrons.
The Casino de Genting is the only casino in Malaysia, though it technically is made up of three individual themed casinos: Monte Carlo Casino, Hollywood Casino, and StarWorld casino. Online casinos are available from operators outside of Malaysia, and we have curated a list of the best ones available to players.
titanium number of valence electrons|Titanium Valence Electrons